Energy, Work and Heat
Introduction
Energy, Work and Heat are three terms that are commonly confused in the study of thermodynamics. Although all three use the same units of joules (J), they are distinct properties connected by the first law of thermodynamics. Many terms are needed for the discussion of thermodynamics, and the definitions will be described below. This exploration will discuss the various forms of energy, work, and heat, their similarities, and their differences.
Definitions:
- System is the components of interest or study
- Open system is a system in which matter and energy in the form of heat or work can be exchanged
- Closed system is one in which energy flows freely but matter is prevented from passing in or out of the system
- Isolated system is one where neither energy nor matter is exchanged
- Surroundings are the components around the system which may influence the system
- State function is a property of a system that is uniquely determined by the present state of a system. Therefore, it is path independent, meaning that the net change is all that matters. The opposite is a path function, where the path of the change affects the total value. The way you get there defines the path.
- Energy (E) is the capacity to do work
- Internal energy (U) is the total energy contained by a thermodynamic system
- Endothermic reaction is when energy is absorbed by the system
- Exothermic reaction is when energy is released from the system to the surroundings
- Work (w) is a path function showing the mechanical energy deposited into a system. In its most basic terms, work is force x distance. For example, work due to gravity is equal to the force of gravity (which is mass x acceleration due to gravity) x distance traveled. There are differing conventions on the ways to define work, but we define work as positive when it is done on the system, as in compression in pressure-volume work. Work by the system is the opposite of work on the system.
- Work done in a system of a solid or a liquid is generally mechanical work. Gases go through mostly pressure-volume work, where expansion is negative work, and compression is positive work. w = - PΔV, as displayed below.
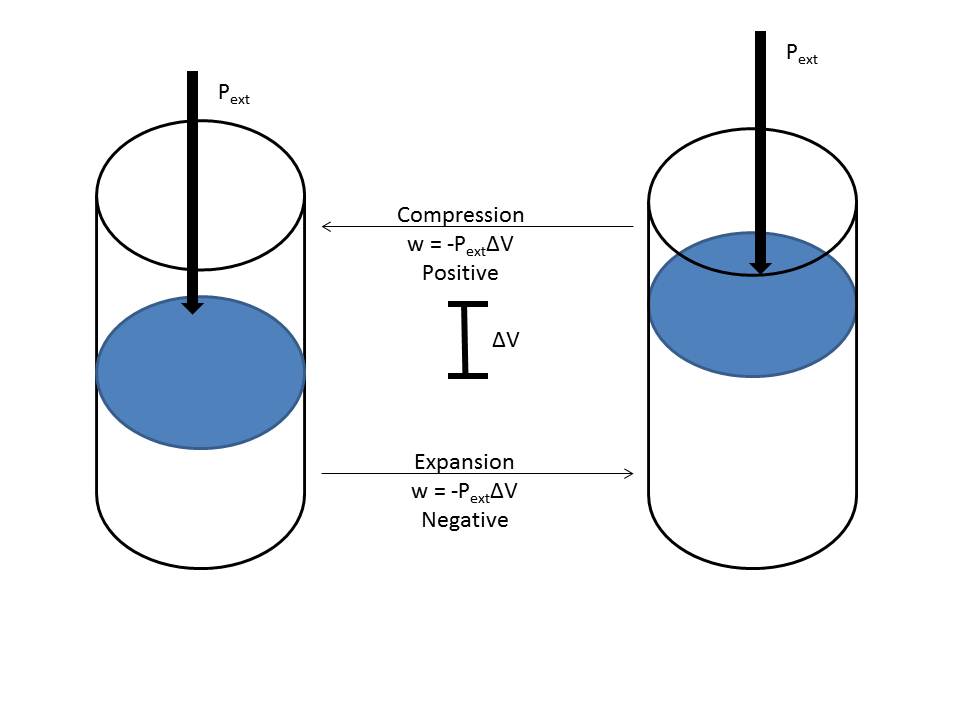
- Heat (q) is a path function showing the energy transferred from one system to another by thermal interaction. We define heat moving into the system as positive, calculated by:
q=m*Cs*(ΔT)
where m is mass, T is temperature, and Cs is defined below. Heat is defined under constant pressure as qp and constant volume as qv. Heat is not the same thing as temperature. Temperature shows the average kinetic energy of a system, where heat is the energy transfer of a system that is not mechanical energy. Heat is not the only thing that can increase temperature - work can also, because work is a form of energy transfer through mechanical means. This is also where the common day usage of terms and scientific usage of terms diverges. For example, when raising the temperature of something in the microwave, there is no heat involved. Instead, radiation work is being done on the system to raise the temperature. Radiation work is mechanical work; therefore it cannot be classified as heat.
- Heat capacity (C) is the measurable physical quantity that characterizes the amount of heat required to change a substance’s by a given amount. All heat capacities for gases can vary if under constant pressure (cp) or constant volume (cv) conditions. Also, the ideal gas law gives us that cp = cv + nRT, where cv depends on the amount of atoms and geometric composition of the gas molecule. The heat capacity at constant pressure is greater because work can occur, while at a constant volume, there can be no work done.
- Specific heat capacity (Cs) is the heat capacity per unit mass of a certain material
- Molar heat capacity (c) is the heat capacity per mole of a certain material
Difference between Energy, Heat, and Work:
Work is an external force on a body times the distance through which the force acts. Therefore, in order for there to be work, there has to be a movement such as a change in volume. Therefore, work is a means of changing internal energy of a macroscopic system through purely mechanical interactions between the systems and its surrounds. Heat, on the other hand, is a means of increasing the internal energy of a system without mechanical interaction. They both affect total energy, just in differing ways. Internal energy is a state function that is the summation of heat and work as seen in the definition:
ΔU = q + w
First Law of Thermodynamics:
The first law of thermodynamics is the change in the internal energy (ΔU) of a system is equal to the work done on it plus the heat transferred to it.
ΔU = q + w
For a system to gain heat (q) it must be gained from its surroundings, therefore the surroundings will lose an equivalent amount of heat. The same applies for work. It follows that:

The implications of the first law are immense. It states that energy cannot be created or destroyed, only conserved between different forms. This is also known as the law of conservation of energy. The total energy in the universe is finite - it can never be changed.
Heat Capacity at a Constant Volume:
When the system is at a constant volume, no work can be done, because ΔV = 0, so ΔU = q. Because qv is equivalent to ΔU, it can be called the energy capacity, representing the capacity of energy change in the system, which is solely due to heat. Energy capacity is just the ability of the system to absorb or give up energy. This is seen in a bomb calorimeter.
Heat Capacity at Constant Pressure: Enthalpy:
Enthalpy is a state function that is the change total change in energy, including the internal energy and work. It is significant because it helps determine the likelihood of a process occurring and the amount of free work possible. Enthalpy also measures the amount of heat created or absorbed in a reaction. We use a normal calorimeter to measure this. Most experiments are run under constant pressure, normally atmospheric.
Under constant pressure situations, so we use the heat capacity at constant pressure. the formulas change because work can affect the system. Work is another way of changing the energy of a system through mechanical means, which affects the enthalpy, as seen below.
From the first law of thermodynamics we have
ΔU = q + w
Work is calculated - PΔV. If we assume the internal pressure is equivalent to the external pressure, as is true under constant pressure conditions, then:

where ΔH is the enthalpy. Pressure is constant, therefore we are interested in the combined change in internal energy and pressure-volume work. This combination of internal energy and pressure-volume is defined as defined by the equation:

Is it important to note that all of these equations apply to constant pressure systems. If pressure changes, the more general solution below must be used:

Questions:
1. A balloon full of nitrogen gas is heated from 30 °C to 100 °C and, as a result, expands. By the convention explained above, what is the sign of w?
a. negative, because work done on the system is negative
b. positive, because work done on the system is negative
c. positive, because work done by the system is negative
d. negative, because work done by the system is negative
2. In an isolated system, such as the universe, the change in internal energy (ΔU), given standard definitions, is:
a. q+w
b. -q
c. 0
d. -w
3. Which of these are apsects of work?
a. Force x distance
b. mass x acceleration due to gravity x distance traveled
c. as path dependent
d. all of the above
Answers:
1. d. Since the gas is expanding, we define work by the system as negative.
2. c. The total energy of the universe never changes.
3. d. These are all forms of and attributes of work.
Citations:
Oxtoby, et al. Principles of Modern Chemistry.
Geva, Eitan. Lecture Slides.
Sension, Roseanne. Lecture Slides.
Comments (0)
You don't have permission to comment on this page.